
Aurora is the name given to the glow or light produced when electrons from space flow down Earth’s magnetic field and collide with atoms and molecules of the upper atmosphere in a ring or oval centered on the magnetic pole of Earth. The collisions produce light much like how electrons flowing through gas in a neon light collide with neon and other gasses to produce different colored light bulbs.
The Aurora is also called the Northern Lights in the northern hemisphere and Southern Lights in the southern hemisphere. The technical term for the Northern Lights is Aurora Borealis and the Southern lights are called the Aurora Australis. The word Aurora was first used by Galileo and comes from Latin and is the name of the goddess of dawn. The word Borealis comes from the Latin word boreal which means “northern” or “from the north”. The word Australis is Latin for austral, which means “southern.”
The aurora is formed from interactions between the solar wind streaming out from the sun and Earth’s protective magnetic field, or magnetosphere. The aurora is one manifestation of geomagnetic activity or geomagnetic storms. As the solar wind increases in speed and the interplanetary magnetic field embedded in the solar wind turns southward, the geomagnetic activity will increase and the aurora will become brighter, more active, and move further from the poles. Even moderate solar wind creates aurora so there is usually a weak aurora somewhere even when there isn’t a big geomagnetic storm.
There are two types of solar events that create big geomagnetic storms that are associated with bright and active aurora. The first is a Coronal Mass Ejection, or CME, which can be described as a billion tons of plasma ejected from the sun, traveling at a million miles per hour. When a CME arrives at Earth, it can produce some of the biggest geomagnetic storms and thus, some of the brightest and most active auroras that extend furthest toward the equator. The second solar event that can create moderate sized geomagnetic storms is called a coronal hole. Coronal holes are the source of high speed solar wind streams. When these high speed streams arrive at Earth, they can produce active auroras. But the geomagnetic storms and aurora associated with coronal holes is less active than those from the biggest and fastest CME's.
One subtle note about the source of the auroral electrons. It is technically not the solar wind electrons that create aurora. When the solar wind is strong and the embedded Interplanetary Magnetic Field (IMF) is aligned opposite Earth’s magnetic field (southward), then there is a transfer of more solar wind energy into the magnetosphere which accelerates more of the magnetospheric electrons down the magnetic field towards Earth. Thus, the auroral electrons come from within Earth’s magnetic magnetosphere. The auroral electrons that create the best aurora in the middle of the night actually come from the tail of the magnetosphere, downstream (away from the sun)
The figure below shows the magnetosphere and the locations where electrons are accelerated (in red). The red region on the right of the figure is where the electrons that produce night-time aurora are accelerated and the source of the processes that generate geomagnetic storms.
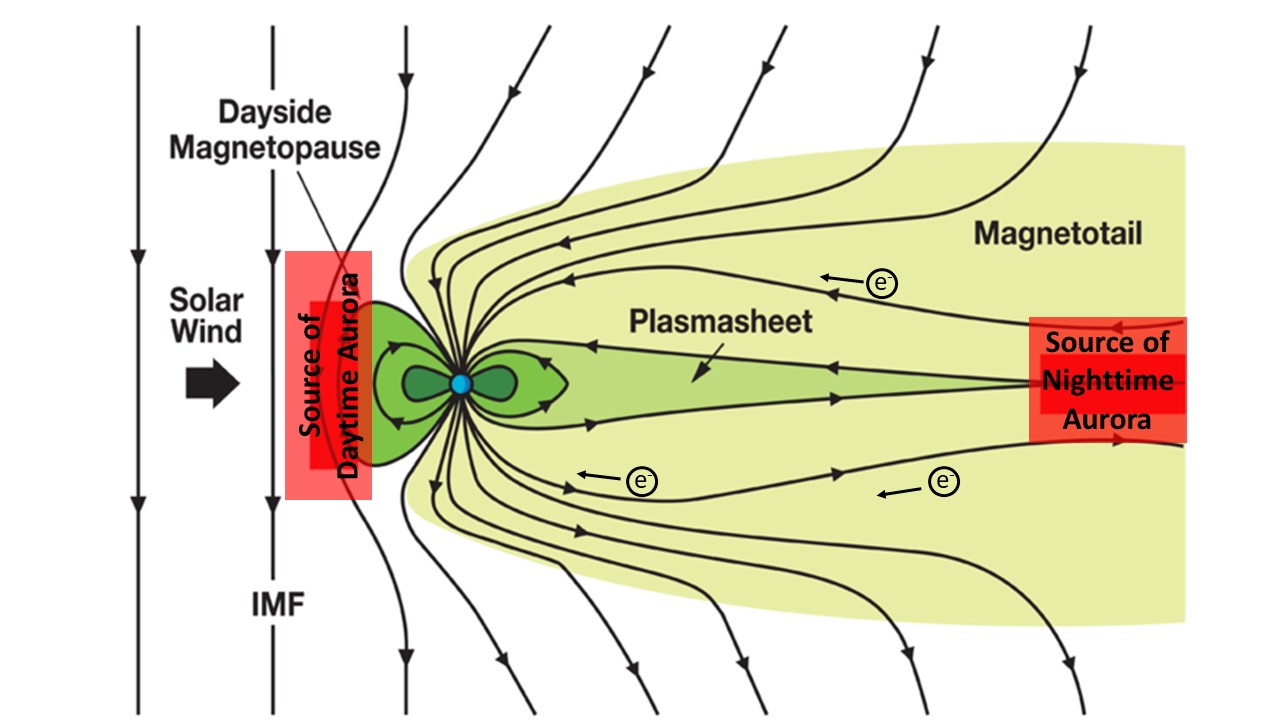
The accelerated electrons follow Earth’s magnetic field lines towards the north and south magnetic poles where they bombard the upper atmosphere and create the aurora. These acceleration regions map down Earth’s magnetic field lines to form an oval of aurora centered on the magnetic poles.
The figure below shows Earth with its magnetic field lines and the electrons traveling down the field lines to the upper atmosphere. The green shaded area shows the general shape of the auroral oval centered on the magnetic pole. The brighter green features show the typical shapes of the aurora in different parts of the auroral oval.
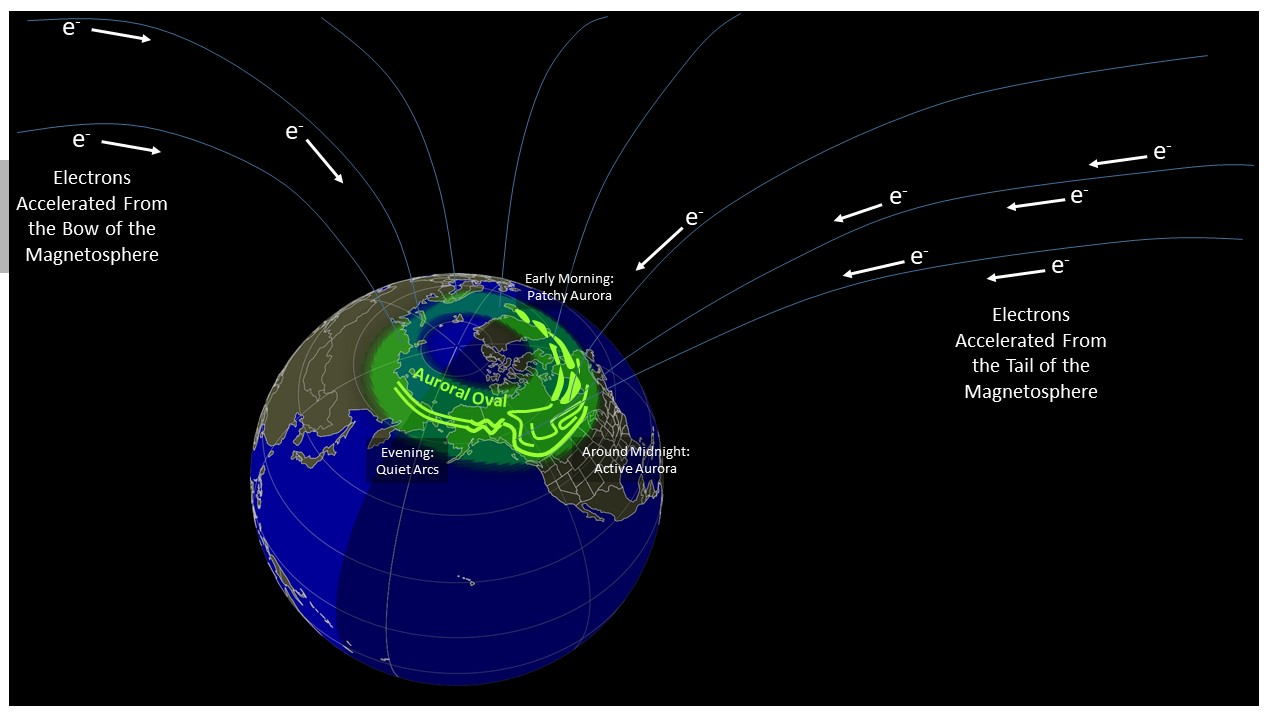
When the fast moving electrons, traveling nearly 1/10th the speed of light (20,000 km/sec or 44 million mph), collide with the atoms and molecules in Earth’s upper atmosphere (above 100 km or 60 miles) they transfer energy to the atmosphere which “excites” the atom or molecule to higher energy states. As the atoms and molecules relax back to their natural energy level, they release photons. These photons are what we see as aurora. This process is similar to the process that occurs in a neon or fluorescent light.
The aurora is one manifestation of geomagnetic activity. The electrons that create the aurora also carry electric currents that are conducted through the ionized portion of the upper atmosphere (the ionosphere). These currents generate perturbations or changes in the magnetic field at the surface of Earth. The changes in the apparent magnetic field of Earth (the geomagnetic field) are measured and are the basis for the creation of geomagnetic disturbance indices such as the Planetary K index, or Kp.
In this way, the geomagnetic index, Kp, is a very good indicator of aurora. The location and the brightness of the aurora are directly correlated with Kp. When the Kp index is high (between 7 and 9), the aurora will be bright and the auroral oval will move to lower latitudes. When the Kp index is moderate (5 or 6), the aurora will retreat towards the poles and be less bright and a little less active. It should be noted however that even lower levels of geomagnetic activity (Kp of 3 or 4) can result in nice aurora with reasonable brightness and activity. The auroral is just going to be closer to the magnetic poles as the auroral oval contracts.
The different colors of the aurora are produced when different atmospheric atoms and molecules are excited to various energy levels. The most common auroral color is a pale green color at a wavelength of 557.7 nm. This is the result of atomic oxygen having been excited to the 1S, or singlet S, state. Another less common color is a deep red which is the result of atomic oxygen having been excited to the 1D, or singlet D, state. Other colors come from other molecules such as nitrogen.
The figure below shows a typical spectrum of aurora.
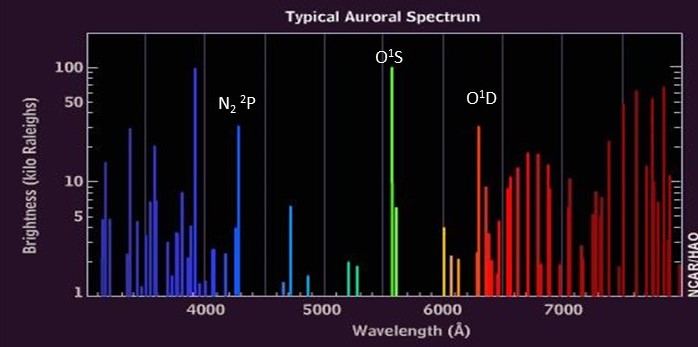
Different auroral colors come from different heights in the atmosphere primarily because the life-time of an excited atom or molecule (time spent in its excited state) is vastly different for different colors of the aurora. The green aurora from oxygen in the 1S state typically occurs from 120 to 400 km (80 to 250 miles) above the surface of Earth. The red aurora from oxygen in the 1D state is restricted to altitudes above 300 km (180 km). This is because oxygen in the 1D state has a very long lifetime (>150 sec) and can only survive in the thinner atmosphere above 300 km. At lower altitudes the oxygen in the 1D state collides with other atmospheric atoms or molecules before it can emit a photon which deactivates or quenches the excited oxygen. The 1S state of oxygen has a lifetime of about 1 second and therefore emits a photon more quickly and thus can emit at lower altitudes where the density is higher. The aurora sometimes has a purplish lower border which comes from emissions from molecular nitrogen. This "prompt" emission is emitted from excited states of nitrogen that have almost no delay between excitation and emission. It survives at even lower altitudes between 120 and 200 km (80 to 120 miles). In the video sequence at the end of this readme document, the purple emissions from the nitrogen seems to lead the green emissions from the oxygen. This is an example of the difference between the prompt emission of nitrogen vs the emission from the longer-lived excited state of oxygen.
The figure below shows different colors of aurora. The left panel shows a red aurora. The middle panel shows the most common green aurora. The right panel shows green aurora with purple lower edges.